A tool to facilitate spirometry quality control assessment using 2019 standards.
Read the official abstract here.
Rationale
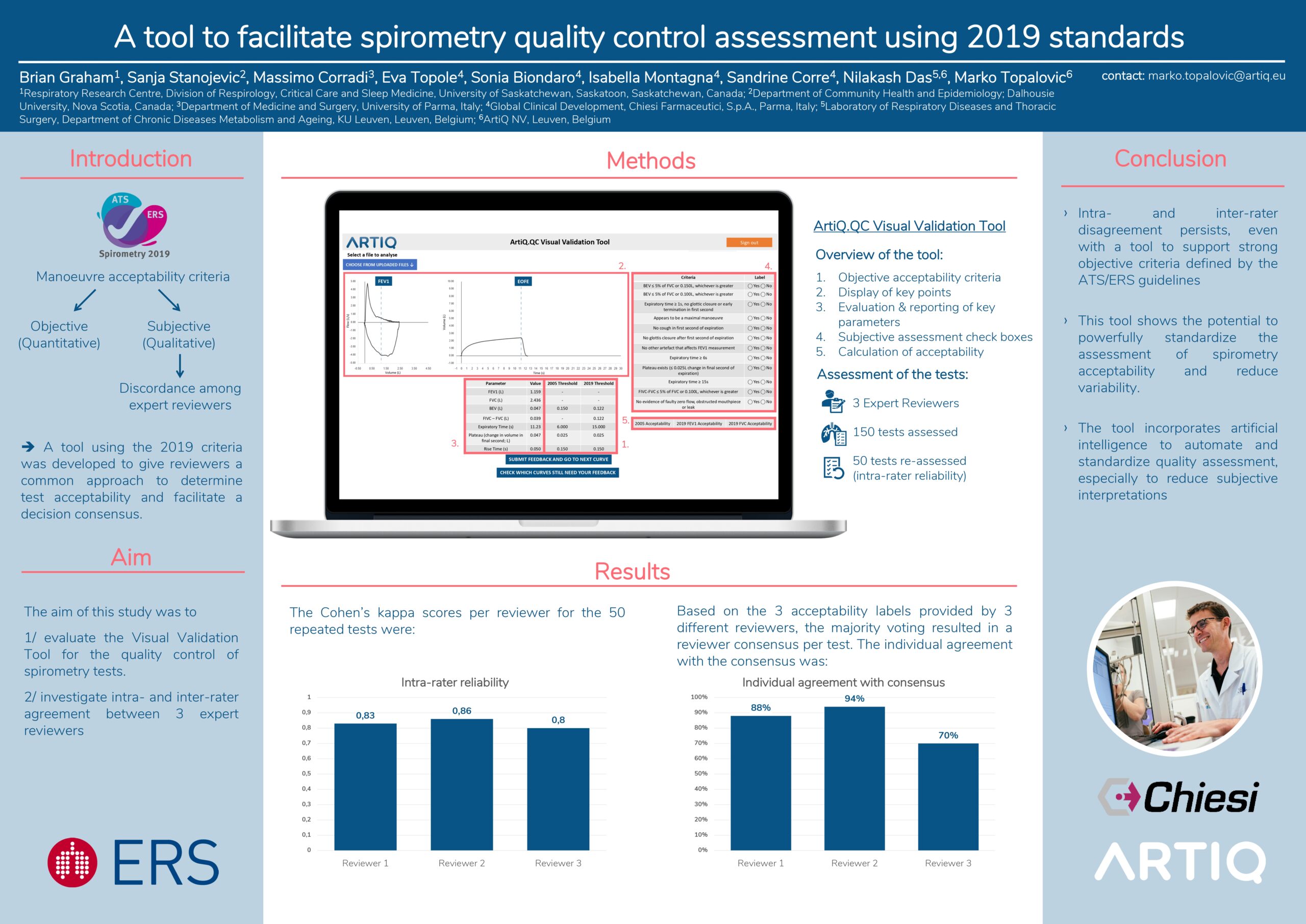
The 2019 ATS/ERS spirometry standards provide objective and subjective manoeuvre acceptability criteria. Subjective components may lead to discordance among expert reviewers. A tool using the 2019 criteria was developed to give reviewers a common approach to determine test acceptability and facilitate a decision consensus.
Methods
The tool applied the objective acceptability criteria, and displayed key points (FEV1, end expiration) on F-V and V-T graphs to aid reviewers in assessing contours and identifying anomalies. The tool evaluates and reports FEV1, FVC, BEV, plateau volume, expiratory time, rise time and inspired volume following EOFE (FIVC). Subjective assessments were: achievement of maximal flow and volume; occurrence of cough, glottis closure or any anomaly in 1st second of expiration; and glottis closure or early termination after 1 s of expiration. Reviewers complete a yes/no check box for each criterion. The tool calculates whether the manoeuvre is acceptable or usable.
Results
Three reviewers used the tool to assess 150 tests. 50 were repeated to assess intra-rater reliability, (Kappa: 0.83, 0.86 and 0.80). All reviewers agreed on 63% of 100 unique tests (Kappa = 0.5). Using the tool to develop reviewer consensus on each test, the individual agreement with the consensus was 88%, 94% and 70%.
Conclusion
Despite using a set of strong objective criteria defined by the spirometry standards, intra- and inter-rater disagreement in assessing spirometry manoeuvre acceptability persists. The developed tool can be used as a powerful way to standardise assessment and reduce variability. The tool incorporates artificial intelligence quality assessment, its additional benefit is being investigated.
Authors
Brian Graham1, Sanja Stanojevic2, Massimo Corradi3, Eva Topole4, Sonia Biondaro4, Isabella Montagna4, Sandrine Corre4, Nilakash Das5,6, Marko Topalovic6
[1] – Respiratory Research Centre, Division of Respirology, Critical Care and Sleep Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, Canada
[2] – Department of Community Health and Epidemiology; Dalhousie University, Nova Scotia, Canada
[3] – Department of Medicine and Surgery, University of Parma, Italy
[4] – Global Clinical Development, Chiesi Farmaceutici, S.p.A., Parma, Italy
[5] – Laboratory of Respiratory Diseases and Thoracic Surgery, Department of Chronic Diseases Metabolism and Ageing, KU Leuven, Leuven, Belgium
[6] – ArtiQ NV, Leuven, Belgium
