Impact on spirometry quality results when using 2005 or 2019 ATS/ERS guidelines
Read the official abstract here.
Introduction
The ATS/ERS 2005 guidelines for standardization of spirometry aimed to guide consistent assessment of all spirograms, to reduce inter-rater variability, and secure reliable measurements. The ATS/ERS spirometry guidelines were updated in 2019, which has the potential to impact spirometry acceptability in ongoing or new clinical trials.
Objectives
This study aims to explore the impact on spirometry curve quality control by using the latest guidelines.
Methods
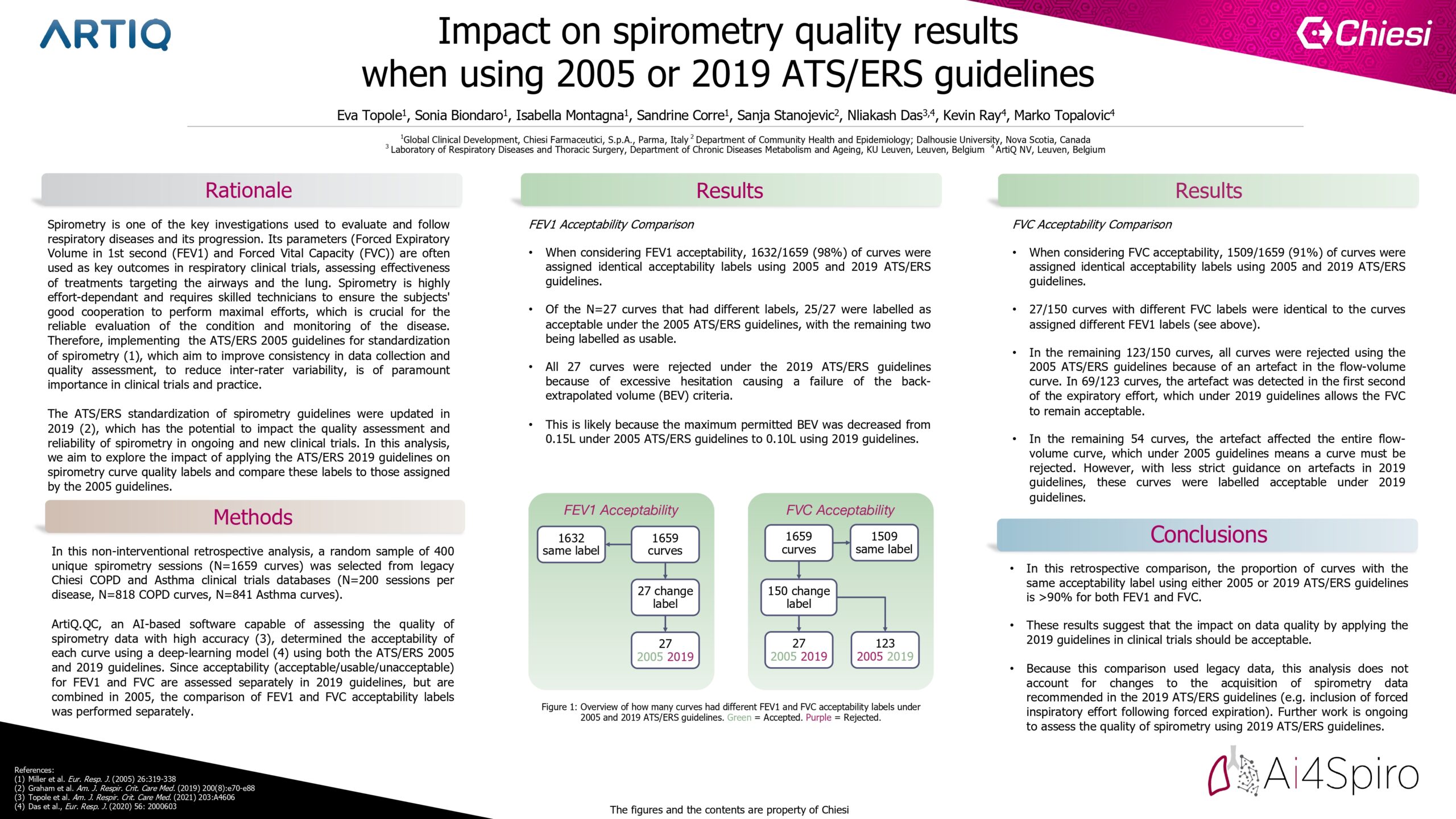
A random sample of 400 spirometry sessions (1659 curves) was selected from legacy Chiesi COPD and Asthma clinical trials database (200 per disease). AI software (ArtiQ.QC) determined the acceptability of each curve using a deep-learning model (Das et al. ERJ 2020) using 2005 and 2019 guidelines. Since acceptability (acceptable/usable/unacceptable) for FEV1 and FVC are assessed separately in 2019 guidelines, but are combined in 2005, the comparison was made separately.
Results
When considering FEV1 acceptability, 98% of curves were equivalent using either guideline. The remaining curves were deemed acceptable by 2005 guidelines, but not by 2019 guidelines, because the back-extrapolated volume (BEV) exceeded the new threshold (0.1L). When considering FVC acceptability, there was agreement in 91% of curves. 2% were rejected by 2019 guidelines because of the more stringent BEV criteria. With less strict guidance on artefacts for FVC in 2019 guidelines, 7% of curves were accepted by 2019 guidelines, but not by 2005.
Conclusion
From this retrospective comparison, there should be minimal impact on data quality by applying the 2019 guidelines. Improvements in data quality may be achieved by appropriate operator training according to 2019 standards.
Authors
Eva Topole1, Sonia Biondaro1, Isabella Montagna1, Sandrine Corre1, Sanja Stanojevic2, Nilakash Das3,4, Kevin Ray4, Marko Topalovic4
Author’s affiliations
1 Global Clinical Development, Chiesi Farmaceutici, S.p.A., Parma, Italy
2 Department of Community Health and Epidemiology; Dalhousie University, Nova Scotia, Canada
3 Laboratory of Respiratory Diseases and Thoracic Surgery, Department of Chronic Diseases Metabolism and Ageing, KU Leuven, Leuven, Belgium
4 ArtiQ NV, Leuven, Belgium
