ArtiQ.QC facilitates spirometry quality control in asthma and COPD clinical trials
Read the official abstract here.
Introduction
Acquiring high quality spirometry data in clinical trials is important, particularly when using FEV1 or FVC as primary endpoints. In addition to quantitative criteria, the ATS/ERS quality control standards include subjective evaluation which introduces inter-rater variability. Within clinical trials, over-readers usually review spirometry curves to ensure data quality. This study explores the value of artificial intelligence-based quality control software (ArtiQ.QC) to determine spirometry quality in clinical trials.
Methods
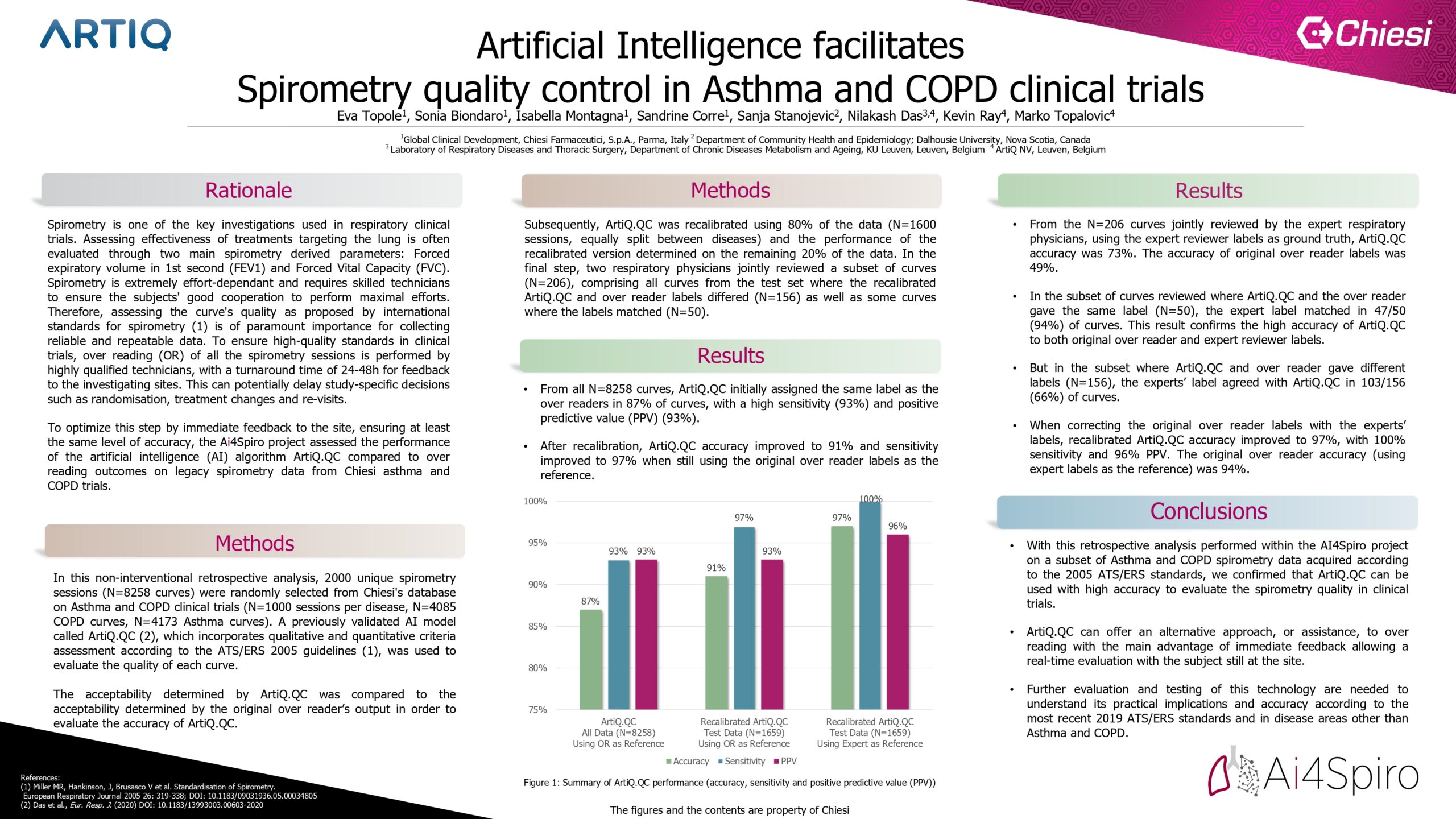
A total of 2000 spirometry sessions (8258 curves) were randomly selected from Chiesi COPD and Asthma clinical trials (1000 sessions per disease). Acceptability using the 2005 ATS/ERS guidelines was determined by over-reader review, and compared with acceptability defined by ArtiQ.QC (Das et al. ERJ 2020). In addition, two respiratory physicians jointly reviewed a subset of curves.
Results
ArtiQ.QC agreed with over-readers in 87% of cases, with 93% sensitivity and 93% positive predictive value (PPV). In a subset of data, when ArtiQ.QC and over-readers agreed, the independent physician review agreed in 47/50 (94%) curves. When ArtiQ.QC and over-reader labels disagreed, the independent physicians agreed with ArtiQ.QC in 103/156 (66%) curves. Inter-rater variability in quality control assessment likely impacts the sensitivity and specificity of the software.
Conclusion
ArtiQ.QC software results are comparable to the over-reader’s and could assist in the quality assessment of spirometry in clinical trials. By providing immediate and consistent results, using ArtiQ.QC may benefit clinical trial conduct and reduce the variability in outcomes.
Authors
Eva Topole1, Sonia Biondaro1, Isabella Montagna1, Sandrine Corre1, Sanja Stanojevic2, Nilakash Das3,4, Kevin Ray4, Marko Topalovic4
Author’s affiliations
1 Global Clinical Development, Chiesi Farmaceutici, S.p.A., Parma, Italy
2 Department of Community Health and Epidemiology; Dalhousie University, Nova Scotia, Canada
3 Laboratory of Respiratory Diseases and Thoracic Surgery, Department of Chronic Diseases Metabolism and Ageing, KU Leuven, Leuven, Belgium
4 ArtiQ NV, Leuven, Belgium
